Device maker Novocure said Friday the Food and Drug Administration approved its first-of-a-kind treatment which fights cancerous brain tumors using electrical energy fields. Get the full story »
Inside these posts: Cancer
Visit our Filed page for categories. To browse by specific topic, see our Inside page. For a list of companies covered on this site, visit our Companies page.
Mesirow CEO James Tyree dies of cancer
James Tyree, the Mesirow Financial chief executive who led the group that took control of Sun-Times Media in 2009, died Wednesday, five months after being diagnosed with stomach cancer.
Tyree had stepped up to save the Chicago Sun-Times when it was floundering in bankruptcy court. He led an investor group that bought it for $26 million. Get the full story »
Hospira profits tumble 40% on quality issues, sales
Shares of Hospira Inc. lost 7 percent of their value after the company said fourth-quarter profits fell nearly 40 percent, as the maker of drugs and devices works to improve product quality in the wake of regulatory issues.
Hospira had lower-than-anticipated fourth quarter sales because it has been unable to deliver products to customers fast enough due largely to “quality enhancement initiatives.” Get the full story »
Cancer blood test coming to market
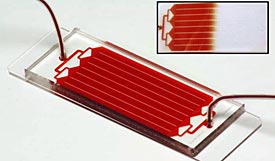
The HB-Chip can spot a single cancer cell lurking among a billion healthy ones . (AP Photo/PNAS Early Edition)
Includes updated information on the latest test format.
A blood test so sensitive that it can spot a single cancer cell lurking among a billion healthy ones is moving one step closer to being available at your doctor’s office.
Boston scientists who invented the test and health care giant Johnson & Johnson will announce Monday that they are joining forces to bring it to market. Four big cancer centers also will start studies using the experimental test this year. Get the full story »
FDA panel rejects Merck drug for prostate cancer
A panel of federal health experts unanimously rejected the use of Merck’s Proscar to prevent prostate cancer, saying the drug could actually raise the risk of the most serious types of tumors. Get the full story »
Mesirow’s Tyree to begin chemotherapy next week
James Tyree, the Mesirow Financial chief executive who last week disclosed that he has stomach cancer, will begin chemotherapy next week.
“I feel pretty good,” he told the Tribune Wednesday night. “I’m sitting here stronger and greater than ever, and it’s stunning that these things are inside me”
In a note to Mesirow workers on Thursday, Tyree said that, over the past week, he has completed additional tests and consulted with several more doctors. Get the full story »
U.S. states settle with Bayer over vitamin claims
Attorneys general in Illinois, Oregon and California said on Tuesday that Bayer AG agreed to a $3.3 million settlement over misleading claims that the drug maker’s vitamins reduced men’s risk of prostate cancer.
Under the terms of the settlement, Bayer cannot make claims that its One A Day Men’s multivitamins can prevent or cure prostate cancer or any other disease without scientific evidence, Illinois Attorney General Lisa Madigan said in a statement.
“When manufacturers like Bayer make marketing claims with insufficient scientific proof behind them, they are misleading consumers,” she said. Get the full story »
Get the full story »
Hospira profits tumble over lost cancer drug sales
Hospira Inc.’s third-quarter profit tumbled 40 percent largely due to the loss of U.S. sales of a generic cancer drug the company cannot sell due to a patent settlement that is keeping the cheaper version off the market.
The Lake Forest-based maker of generic injectable drugs and medication delivery devices said net income fell to $71.4 million, or 42 cents a share in the third quarter ended Sept. 30. That compares to $116.2 million, or 71 cents in the third quarter of 2009. Get the full story »
Sun-Times’ Tyree diagnosed with stomach cancer
James Tyree, the Mesirow Financial chief executive who nearly four years ago had a kidney and pancreas transplant, has been diagnosed with stomach cancer.
Tyree said he doesn’t know the stage of the cancer yet.
“I’ll find that out over the next few days,” he told the Tribune in a phone interview from his office, where he continues to work every day. “They did more tests to find out if it has spread anywhere else,” he said, noting that it’s currently in his stomach and one lymph node. Get the full story »
Neopharm narrows losses but needs new funding
Lake Bluff biotech company Neopharm Inc. said Thursday that it had narrowed its losses in both the second quarter and first half of this year and reported progress in clinical trials for its breast, pancreatic and prostate cancer drugs.
But Aquilur Rahman, president and chief executive officer, warned that with cash dwindling to $1.8 million as of June 30, the company needs new funding to continue. Get the full story »
MD Anderson, Advocate may partner in Chicago
MD Anderson Cancer Center, one of the nation’s best known providers of oncology care, is in talks with Chicago’s largest provider of medical care, Advocate Health Care, about a possible partnership, the two health care systems confirmed today.
Any deal with the internationally known MD Anderson would be designed to enhance the cancer care provided at Advocate, which owns and operates 10 hospitals in Illinois, including eight in the Chicago area, according to sources close to the talks. It could also be a marketing opportunity given the MD Anderson name is well-known and regularly ranks atop lists of U.S. cancer providers such as the annual rankings by U.S. News & World Report. Get the full story »
APP to market breast cancer treatment
Schaumburg-based APP Pharmaceuticals Inc. will begin marketing a breast cancer treatment medication in the U.S. as Anastrozole, the drug’s generic name. Anastrozole tablets, also known as Arimidex , are mainly used to treat early breast cancer in post-menopausal women.
APP’s parent company, Fresenius Kabi Pharmaceuticals Holding Inc., said the move comes after the U.S. Food and Drug Administration granted its research company permission to market the drug.





