March 14 at 1:39 p.m.
Filed under:
Pharmaceuticals
By Reuters
(Handout)
Prices for U.S. prescription drugs rose at a faster rate than costs for other medical goods and services in the last four years, according to a new U.S. government report.
The nonpartisan Government Accountability Office found that the “usual and customary” price index for the top 100 commonly used drugs rose an annual average of 6.6 percent from 2006 through the first quarter of 2010, compared with a 3.8 percent average annual increase in the consumer price index for medical goods and services. Get the full story »
Jan. 7 at 2:26 p.m.
Filed under:
Litigation,
Pharmaceuticals,
Technology
By Reuters
The U.S. Supreme Court said Friday that it would decide whether a state law restricting commercial access to information about prescription drug records violated constitutional free-speech rights. Get the full story »
Nov. 15, 2010 at 9:25 a.m.
Filed under:
Pharmaceuticals,
Stock activity
By Reuters
A once-daily pill being developed by Bayer AG and Johnson & Johnson was better at preventing stroke than standard treatment, with less risk of the most worrisome types of bleeding, researchers said on Monday. Get the full story »
Oct. 26, 2010 at 11:54 a.m.
Filed under:
Earnings,
Health care,
Pharmaceuticals
By Associated Press
Bristol-Myers Squibb Co. posted a slight decline in third-quarter profit Tuesday as its restrained spending was offset by flat sales of its drugs, lower income from its partners and bigger discounts to government because of the health care overhaul.
Oct. 8, 2010 at 3:20 p.m.
Filed under:
Earnings,
Pharmaceuticals,
Updated
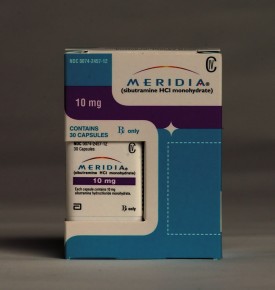 By Bruce Japsen and Andrew Zajac | Abbott Laboratories said Friday that it would withdraw the diet drug Meridia at the request of the U.S. Food and Drug Administration less than a month after it failed to win over one of the federal agency’s safety advisory panels.
By Bruce Japsen and Andrew Zajac | Abbott Laboratories said Friday that it would withdraw the diet drug Meridia at the request of the U.S. Food and Drug Administration less than a month after it failed to win over one of the federal agency’s safety advisory panels.
The FDA confirmed the North Chicago-based drug giant’s decision, saying Abbott withdrew the drug because of “clinical trial data indicating an increased risk of heart attack and stroke.” Get the full story »
Sep. 28, 2010 at 10:10 a.m.
Filed under:
Earnings,
Pharmaceuticals,
Retail,
Updated
By Bruce Japsen

Shoppers at a Walgreens in Vernon Hills. (Lane Christiansen/Chicago Tribune)
Walgreen Co. fourth-quarter profits were up 8 percent in the company’s fourth quarter on prescription sales but impacted somewhat by a large New York pharmacy acquisition earlier this year.
The Deerfield-based company, which operates more than 8,000 stores across the country, this morning reported profits of $470 million, or 49 cents a share in its fourth-quarter ended Aug. 31. That compares to $436 million, or 44 cents a share, in the company’s fourth quarter of last year.
The earnings report helped boost Walgreens shares up nearly 9 percent, or $2.71 a share, to $33.06 in trading this morning on the New York Stock Exchange. Get the full story »
Sep. 17, 2010 at 2:30 p.m.
Filed under:
Health care,
Pharmaceuticals,
Retail
By Associated Press
Prescriptions increased in the second quarter for some of the largest U.S. drugstores and pharmacy benefits managers despite the weak economy, an analyst for Fitch Ratings said Friday.
Analyst Bob Kirby said prescriptions for Medco Health Solutions Inc. and Express Scripts Inc. and for the drugstores Walgreen Co. and CVS Caremark Corp. rose 5.2 percent from a year earlier. Kirby said he expects similar growth for the rest of the year assuming economic conditions don’t change much. Get the full story »
Aug. 31, 2010 at 11:27 a.m.
Filed under:
Pharmaceuticals,
Regulations
By Associated Press
Federal health regulators have issued a warning letter to Baxter International Inc. for exaggerating the benefits of its lung drug in brochures to physicians.
The Food and Drug Administration letter cites the Deerfield, Ill.-based company for making “misleading efficacy claims” in promotional materials for its drug Aralast.
Aug. 23, 2010 at 2:51 p.m.
Filed under:
Pharmaceuticals
By Associated Press
Drug and medical device maker Hospira Inc. said Japanese regulators have approved the use of its sedative Precedex in patients for more than 24 hours. The drug was originally approved there in 2004 to sedate intubated and mechanically ventilated patients in intensive care for up to 24 hours.
A Hospira spokesman said the Lake Forest, Ill., company has done trials of the sedative in the United States, where it also is seeking approval for the longer-term use.
June 24, 2010 at 3:51 p.m.
Filed under:
Pharmaceuticals,
Regulations
By Associated Press
Drug and device maker Abbott Laboratories said Thursday it received U.S. regulatory approval for its Freestyle Lite blood sugar testing strips.
The Food and Drug Administration approved the products to test for levels of glucose, a type of blood sugar, in patients with type 2 diabetes. People with the disease have trouble breaking down carbohydrates because their bodies have become resistant to a protein used in metabolism. Get the full story »
June 18, 2010 at 11:52 a.m.
Filed under:
Pharmaceuticals,
Retail
By Michael Oneal
It turns out Walgreen Co. and CVS Caremark Corp. need each other after all.
After months of contract negotiations, punctuated by a two-week public brawl, the two drug store giants announced Friday that they have settled a dispute that threatened to prevent thousands of people from getting their prescriptions filled at Walgreens stores.
At issue: How Caremark, one of the nation’s biggest prescription plan operators, prices discounts for prescriptions filled at Walgreens pharmacies, which often compete fiercely with CVS stores on nearby street corners around the United States. Get the full story »
April 16, 2010 at 4:44 p.m.
Filed under:
Litigation,
Pharmaceuticals,
Politics
From Crain’s Chicago Business | An ongoing lawsuit against CVS Caremark Corp. is making things awkward for the company as it tries to secure a contract to manage drugs for the city of Chicago. The lawsuit says CVS fraudulently removed labels from prescription drugs for 400,000 Illinois workers,
retirees and their families, and then resold the drugs to the customers.
Get the full story: chicagobusiness.com.







