Oct. 29, 2010 at 5:36 a.m.
Filed under:
Health care,
Pharmaceuticals,
Policy
By Reuters
Vivus Inc.’s weight-loss drug candidate Qnexa failed to win over U.S. health regulators, who declined to approve the diet pill, asking for evidence related to heart risk and other information.
The Food and Drug Administration told Vivus on Thursday that its new drug application for Qnexa could not approved in its present form. Get the full story »
Oct. 8, 2010 at 3:20 p.m.
Filed under:
Earnings,
Pharmaceuticals,
Updated
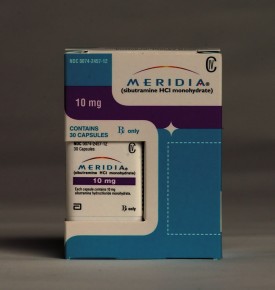 By Bruce Japsen and Andrew Zajac | Abbott Laboratories said Friday that it would withdraw the diet drug Meridia at the request of the U.S. Food and Drug Administration less than a month after it failed to win over one of the federal agency’s safety advisory panels.
By Bruce Japsen and Andrew Zajac | Abbott Laboratories said Friday that it would withdraw the diet drug Meridia at the request of the U.S. Food and Drug Administration less than a month after it failed to win over one of the federal agency’s safety advisory panels.
The FDA confirmed the North Chicago-based drug giant’s decision, saying Abbott withdrew the drug because of “clinical trial data indicating an increased risk of heart attack and stroke.” Get the full story »
Sep. 16, 2010 at 2:27 p.m.
Filed under:
Government,
Pharmaceuticals
By Bruce Japsen
An advisory panel to the Food and Drug Administration voted against recommending an experimental diet drug known as lorcaserin be approved for marketing.
In a first of several votes to be taken this afternoon, only five members of the panel voted that the drug’s potential benefits to overweight Americans outweigh risks. Nine voted against the drug’s benefits-to-risks ratio. Get the full story »
Sep. 15, 2010 at 3:17 p.m.
Filed under:
Pharmaceuticals
By Bruce Japsen
In a rebuke of the safety of Abbott Laboratories diet drug Meridia, eight of 16 members of a U.S. Food and Drug Administration advisory panel said this afternoon that the drug should be withdrawn from the U.S. market.
Meanwhile, six of the panelists said the drug should only be prescribed by “specially trained physicians” and include a strict FDA black box warning noting the new limits.
The other two panelists also said a new boxed warning should be added to alert consumers of increased risks of heart attacks and closer monitoring of patients by clinicians. Get the full story »
Sep. 15, 2010 at 5:45 a.m.
Filed under:
Health care,
Pharmaceuticals
By Bruce Japsen
A Food and Drug Administration advisory panel will decide Wednesday whether the diet drug Meridia will remain on the market amid calls that it be removed. And on Thursday, another drug, known as lorcaserin, is up before an advisory committee where its developer will face questions from panelists and a possible recommendation for agency approval.
Sep. 2, 2010 at 7:06 a.m.
Filed under:
Pharmaceuticals
By Tribune newspapers
The prescription diet drug sibutramine, sold under the brand name Meridia, should be taken off the market because it raises the risk of heart attacks and strokes in some patients, the editor of the New England Journal of Medicine said Wednesday.
Those risks, published in January on a government clinical-trials Web site and now in full in the journal, outweigh the modest benefits of the medication, said Dr. Gregory Curfman, the journal’s executive editor and lead author of an editorial that accompanied the study.
Sep. 1, 2010 at 5:46 p.m.
Filed under:
Consumer news,
Health care,
Pharmaceuticals,
Policy
By Los Angeles Times
The prescription diet drug sibutramine, sold under the brand name Meridia, should be taken off the market because it raises the risk of heart attacks and strokes in some patients, the editor of the New England Journal of Medicine said Wednesday.
Those risks, published in January on a government clinical-trials website and now in full in the journal, outweigh the modest benefits of the medication, said Dr. Gregory D. Curfman, the journal’s executive editor and lead author of an editorial that accompanied the study. Get the full story »
Aug. 6, 2010 at 1:12 p.m.
Filed under:
By Reuters
Abbott Laboratories’ weight loss drug Meridia will be reviewed by an advisory panel to the U.S. Food and Drug Administration on Sept. 15, the company said Friday. Get the full story »





