Jan. 21 at 7:48 a.m.
Filed under:
Government,
Pharmaceuticals
By Dow Jones Newswires
Vivus Inc. said Friday the Food and Drug Administration has asked the drug developer to assess the feasibility of a study on whether an ingredient in the weight-loss drug Qnexa causes birth defects.
The request represents another setback for the drug intended to treat obesity. Vivus and rival Arena Pharmaceuticals Inc. had their diet drugs rejected by the agency in October, as competitor Orexigen Therapeutics Inc.’s weight-loss drug Contrave continued to move through the approval process. Get the full story »
Oct. 8, 2010 at 3:20 p.m.
Filed under:
Earnings,
Pharmaceuticals,
Updated
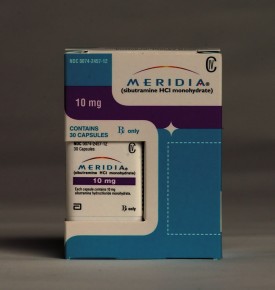 By Bruce Japsen and Andrew Zajac | Abbott Laboratories said Friday that it would withdraw the diet drug Meridia at the request of the U.S. Food and Drug Administration less than a month after it failed to win over one of the federal agency’s safety advisory panels.
By Bruce Japsen and Andrew Zajac | Abbott Laboratories said Friday that it would withdraw the diet drug Meridia at the request of the U.S. Food and Drug Administration less than a month after it failed to win over one of the federal agency’s safety advisory panels.
The FDA confirmed the North Chicago-based drug giant’s decision, saying Abbott withdrew the drug because of “clinical trial data indicating an increased risk of heart attack and stroke.” Get the full story »
Sep. 15, 2010 at 5:45 a.m.
Filed under:
Health care,
Pharmaceuticals
By Bruce Japsen
A Food and Drug Administration advisory panel will decide Wednesday whether the diet drug Meridia will remain on the market amid calls that it be removed. And on Thursday, another drug, known as lorcaserin, is up before an advisory committee where its developer will face questions from panelists and a possible recommendation for agency approval.





